Part:BBa_J45199:Experience
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_J45199
Banana odor based reporter of transcriptional signal.
User Reviews
UNIQ8321c0aa679ecee0-partinfo-00000000-QINU
|
••••• |
BBa_J45199 was successfully used to produce banana odor under the control of BBa_R0040 and BBa_J45992in the banana odor generators BBa_J45200 and BBa_J45250, respectively. |
UNIQ8321c0aa679ecee0-partinfo-00000007-QINU
Characterization
Characterization of Constituively Expressed and Arsenic Induced ATF1 by WPI iGEM
To test the efficiency of the ATF1 enzyme when produced constitutively and when regulated by the arsenic inducible promoter , we grew a 50 mL culture of E. coli transformed with BBa_K1423006 and a 10 mL culture of E. coli transformed with BBa_K1423007 overnight in a 37°C shaker, then diluted the cultures to OD 0.2 the following day. 2 mL of the diluted liquid culture trasnformed with the arsenic inducible construct were then added to 24 test tubes to create smaller liquid cultures. For the constitutive construct, 6 2mL liquid cultures were made from the diluted stock. Varying concentrations of isoamyl alcohol and sodium arsenite were added to the 2mL cultures. The contents of each test tube are outlined below. Note that three cultures were made for tubes 1-10.
1 a-c. 2mL E. coli transformed with BBa_K1423007
2 a-c. 2mL E. coli transformed with BBa_K1423007 + 5mM Isoamyl Alcohol
3 a-c. 2mL E. coli transformed with BBa_K1423006
4 a-c. 2mL E. coli transformed with BBa_K1423006 + 5mM Isoamyl Alcohol
5 a-c. 2mL E. coli transformed with BBa_K1423006 + 100 μM sodium arsenite
6 a-c. 2mL E. coli transformed with BBa_K1423006 + 5mM Isoamyl Alcohol + 0.5 μM sodium arsenite
7 a-c. 2mL E. coli transformed with BBa_K1423006 + 5mM Isoamyl Alcohol + 5 μM sodium arsenite
8 a-c. 2mL E. coli transformed with BBa_K1423006 + 5mM Isoamyl Alcohol + 10 μM sodium arsenite
9 a-c. 2mL E. coli transformed with BBa_K1423006 + 5mM Isoamyl Alcohol + 50 μM sodium arsenite
10 a-c. 2mL E. coli transformed with BBa_K1423006 + 5mM Isoamyl Alcohol + 100 μM sodium arsenite
11. 2mL LB + 5mM Isoamyl Alcohol + 5 mM Isoamyl acetate (standard)
12. 2mL 2x dilution of the standard
13. 2mL 10x dilution of the standard
14. 2mL 25x dilution of the standard
15. 2mL 50x dilution of the standard
The cultures above were parafilmed to prevent the evaporation of the isoamyl acetate product and were grown overnight in a 37°C shaker. Following the overnight incubation, 1 mL of each liquid culture was transfered into a gas chromatography vial. A gas chromatography/mass spectroscopy experiment was run to quantify the efficiency of the ATF1 enzyme. The conversion of isoamyl alcohol to isoamyl acetate was calculated by measuring the area under the curve in the gas chromatograph. The results of this experiment can be seen by clicking the link below.
Cultures induced with 0.5 μM, 5 μM, and 10 μM sodium arsenite showed increased yield of isoamyl acetate over cultures that constitutively converts isoamyl alcohol to isoamyl acetate. Arsenite concentrations of 0.5 μM and 5 μM resulted in a yield that was over 3 times greater than that for the constitutive conversion to isoamyl acetate. The cultures induced with 10 μM arsenite produced about 2.5 times more isoamyl acetate than the culture constituitively expressing ATF1. Beyond sodium arsenite concentrations of 10 μM, isoamyl acetate production decreased because the cells could not withstand the high concentrations of arsenite.
Smell test of banana odor generator
The banana odor generator (BBa_J45200) includes the ATF1 enzyme generator (BBa_J45199) under the control of a constitutive promoter (BBa_R0040).

At the 2006 iGEM Jamboree, we conducted blind smell tests in which iGEM participants had to distinguish between cell cultures producing wintergreen odor, banana odor and the natural fecal odor of E. coli. Of the 116 respondents, 64% were able to correctly identify the culture producing methyl salicylate through its wintergreen bouquet, 87% were able to correctly identify the culture producing isoamyl acetate through its banana bouquet, and 86% were able to correctly identify the laboratory E. coli strain TOP10 through its stink. The error bars are the specific margin of error at 95% confidence. For the smell tests, we used the odor-free chassis (E. coli strain YYC912) for the wintergreen and banana odor generators.
Gas chromatography analysis of banana odor generator
The banana odor generator (BBa_J45200) includes the ATF1 enzyme generator (BBa_J45199) under the control of a constitutive promoter (BBa_R0040).

We successfully designed, constructed and tested biosynthetic devices for banana odor production from exogenously supplied precursor. To confirm that the banana odor generator produced the correct smell compound, we analyzed cultures with the device supplemented with 5mM isoamyl alcohol by gas chromatography. The banana odor generator (BBa_J45200) produced high levels of isoamyl acetate when we added the precursor isoamyl alcohol to the culture medium (A). The cellular chassis alone (E. coli strain TOP10) did not produce isoamyl acetate although we added isoamyl alcohol to the culture medium (B). The retention time of the isoamyl acetate peak from BBa_J45200 is identical to that of the pure isoamyl acetate standard (C). Most E. coli strains produce indole. Octyl acetate was used as an internal standard for all samples containing isoamyl acetate.
Use of ATF1 generator in banana odor generator
The ATF1 enzyme generator (BBa_J45199) is used in both banana odor generators (BBa_J45200 and BBa_J45250).
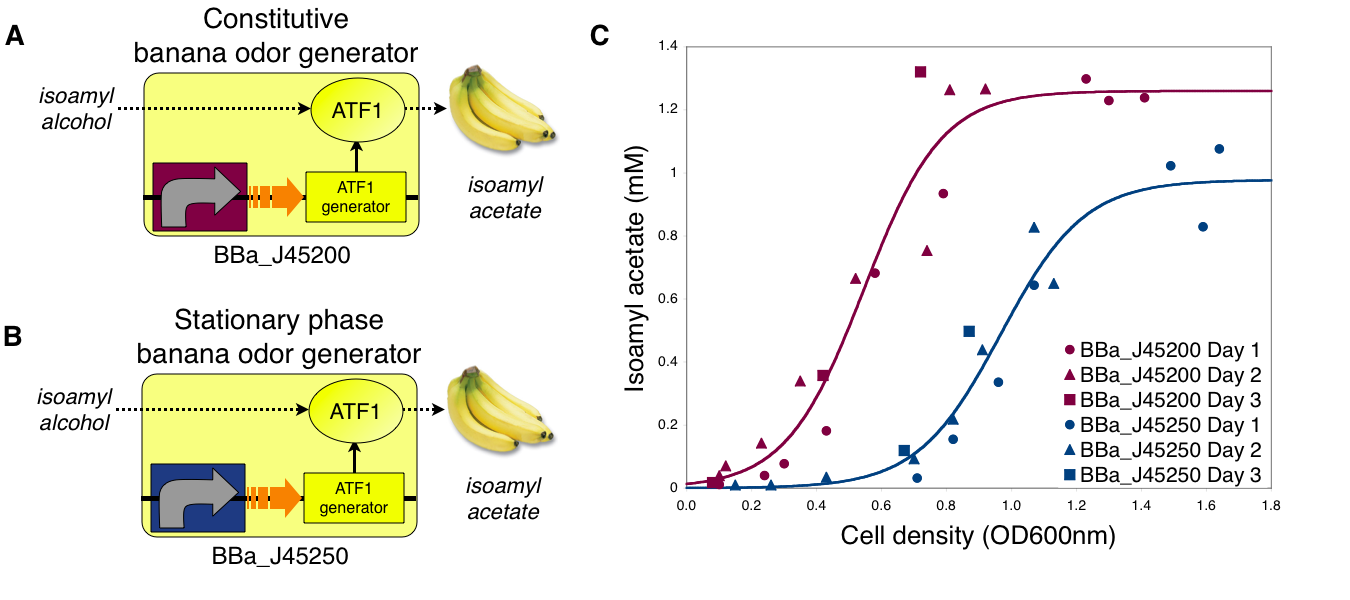
To demonstrate growth phase dependent banana odor production, we compared the behavior of constitutive and stationary phase dependent banana odor generators (A and B, respectively). We measured isoamyl acetate concentrations of cultures of the constitutive and stationary phase banana odor generators at different culture cell densities (OD600nm) (C). As expected, the stationary phase banana odor generator produced very little isoamyl acetate at low cell densities but its isoamyl acetate production increased with cell density. By comparison, the constitutive banana odor generator produced more isoamyl acetate at lower cell densities than the stationary phase banana odor generator. To visually aid comparison of the two odor generators, an empirical fit to the data for each device is shown.

 1 Registry Star
1 Registry Star